



Next: 7 Basic Rules for
Up: EPR Tutorial
Previous: 5.2.3 Tetracyanoethene anion radical
Contents
When a radical present 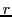 different groups of equivalent nuclei with spin
different groups of equivalent nuclei with spin 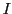 ,
it will have different coupling constants,
,
it will have different coupling constants, 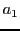 ,
, 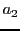 ,
, 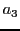 ,
,  ,
, 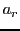 ,
and it can give a complex hyperfine structure.
The values of
,
and it can give a complex hyperfine structure.
The values of 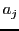 can be obtained for direct measure on the spectrum.
can be obtained for direct measure on the spectrum.
The position of all lines in the spectrum is given by:
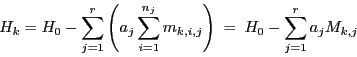 |
(7) |
where 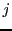 indicates the groups of equivalent nuclei and
indicates the groups of equivalent nuclei and 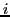 the nuclei within each group.
the nuclei within each group.
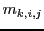 are the individual Z components of the angular momentum of nuclear spin
are the individual Z components of the angular momentum of nuclear spin 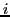 of the equivalent group
of the equivalent group 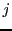 in the state
in the state 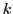 and
and 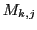 is the total Z component of the angular momentum
of spin of the equivalent nuclei
is the total Z component of the angular momentum
of spin of the equivalent nuclei 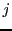 in the state
in the state 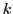 .
.
The spectrum of a neutral radical derived from the methanol
(obtained by abstraction of an atom of H in the photolysis of a dissolution of 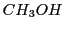 and
and 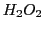 )
)
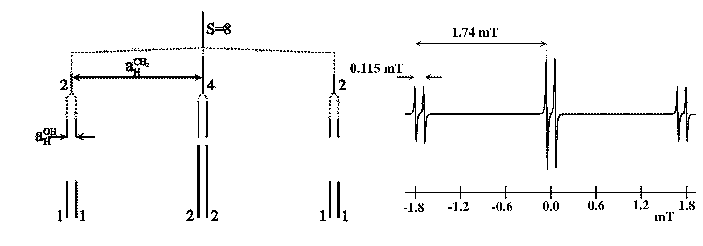
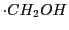 is shown in Fig. 15.
The electron is interacting with three protons, two of which are equivalent.
The smallest splitting corresponds to the proton of the OH.
Three doublets are obtained with a relation of intensities 1:2:1.
The values of the two coupling constants and the reconstruction of the spectrum are also
indicate in the Fig. 15.
is shown in Fig. 15.
The electron is interacting with three protons, two of which are equivalent.
The smallest splitting corresponds to the proton of the OH.
Three doublets are obtained with a relation of intensities 1:2:1.
The values of the two coupling constants and the reconstruction of the spectrum are also
indicate in the Fig. 15.
Figure 15:
Successive splittings and EPR spectrum of the 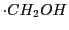 .
.
 |




Next: 7 Basic Rules for
Up: EPR Tutorial
Previous: 5.2.3 Tetracyanoethene anion radical
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada
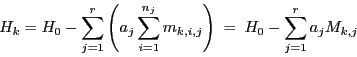
![]() and
and ![]() )
)
![]() is shown in Fig. 15.
The electron is interacting with three protons, two of which are equivalent.
The smallest splitting corresponds to the proton of the OH.
Three doublets are obtained with a relation of intensities 1:2:1.
The values of the two coupling constants and the reconstruction of the spectrum are also
indicate in the Fig. 15.
is shown in Fig. 15.
The electron is interacting with three protons, two of which are equivalent.
The smallest splitting corresponds to the proton of the OH.
Three doublets are obtained with a relation of intensities 1:2:1.
The values of the two coupling constants and the reconstruction of the spectrum are also
indicate in the Fig. 15.