



Next: 5.2.1 Di-tert-butyl nitroxide neutral
Up: 5 Radical Spectra with
Previous: 5.1.7 Cyclooctatetraene anion radical
Contents
5.2 Radicals that contain n equivalent nuclei with I = 1
The interaction of a nucleus with 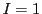 (
(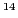 N,
N, 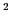 H) with an unpaired electron,
will give three lines of equal height (1:1:1), called triplet.
The position of these three lines is obtained replacing the three
H) with an unpaired electron,
will give three lines of equal height (1:1:1), called triplet.
The position of these three lines is obtained replacing the three 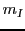 values (
values (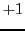 ,
, 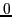 and
and 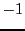 )
in Eq. (5):
)
in Eq. (5):
The distance in mT between two consecutive lines is the value of the hyperfine splitting a.
If a radical contains two equivalent nuclei with I = 1, the number of lines is five
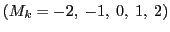 with the relative intensities (1:2:3:2:1), called quintet.
The position of each line, Eq. (6) is:
with the relative intensities (1:2:3:2:1), called quintet.
The position of each line, Eq. (6) is:
The EPR spectrum for a substituted nitronyl nitroxide radical
and the corresponding splitting are presented in Fig. 11.
The numbers written on the splitting lines indicate the relative degeneration (height of the lines).
The hyperfine splitting value of the two equivalent nitrogen atoms is also indicated in Fig. 11.
Figure 11:
Successive splittings and EPR spectrum of a substituted nitronyl nitroxide.
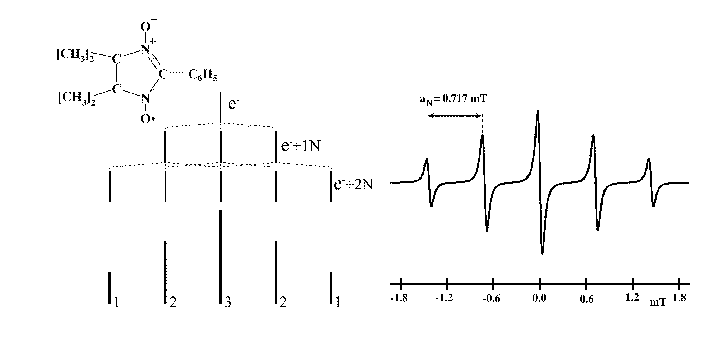 |
Table 3:
Relative intensities observed for the lines generated by n nuclei with I = 1.
| n |
Relative intensity |
N |
S |
| 1 |
|
|
|
|
1 |
1 |
1 |
|
|
|
|
3 |
3 |
| 2 |
|
|
|
1 |
2 |
3 |
2 |
1 |
|
|
|
5 |
9 |
| 3 |
|
|
1 |
3 |
6 |
7 |
6 |
3 |
1 |
|
|
7 |
27 |
| 4 |
|
1 |
4 |
10 |
16 |
19 |
16 |
10 |
4 |
1 |
|
9 |
81 |
| 5 |
1 |
5 |
15 |
30 |
45 |
51 |
45 |
30 |
15 |
5 |
1 |
11 |
243 |
Table 4:
Relative intensities observed for the lines generated by n nuclei with I = 3/2.
| n |
Relative intensity |
N |
S |
| 1 |
|
|
|
|
|
|
|
|
|
1 |
|
1 |
|
1 |
|
1 |
|
|
|
|
|
|
|
|
|
4 |
4 |
| 2 |
|
|
|
|
|
|
1 |
|
2 |
|
3 |
|
4 |
|
3 |
|
2 |
|
1 |
|
|
|
|
|
|
7 |
16 |
| 3 |
|
|
|
1 |
|
3 |
|
6 |
|
10 |
|
12 |
|
12 |
|
10 |
|
6 |
|
3 |
|
1 |
|
|
|
10 |
64 |
| 4 |
1 |
|
4 |
|
10 |
|
20 |
|
31 |
|
40 |
|
44 |
|
40 |
|
31 |
|
20 |
|
10 |
|
4 |
|
1 |
13 |
25 |
A series of EPR spectra of radicals in increasing order of difficulty is presented below.
They have n equivalent nuclei (n = 1, 2 and 4) with spin I = 1.
You have to carry out the following operations (similar to those done with the previous examples):
- Click on the corresponding simulator link.
- Measure the values of hyperfine splitting in the experimental spectrum
- Use the value of the hyperfine splitting to simulate the spectrum.
- When you think that the simulation is correct overlap both spectra, the simulated and the experimental,
if you note differences, refine the splitting and/or
change the peak to peak linewidth (see Appendix A).
- Measure the heights of each line and divide the result by the value of the first line (the smallest one).
- Observe the number of equivalent nuclei and the multiplet generated by them.
Compare the results obtained with the data in Table 3.
- Print out the simulated and experimental spectra with the parameters used in the simulation.
When finish, close the window of the simulator to go back to the tutorial.
Subsections




Next: 5.2.1 Di-tert-butyl nitroxide neutral
Up: 5 Radical Spectra with
Previous: 5.1.7 Cyclooctatetraene anion radical
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada
![]() with the relative intensities (1:2:3:2:1), called quintet.
The position of each line, Eq. (6) is:
with the relative intensities (1:2:3:2:1), called quintet.
The position of each line, Eq. (6) is: