Next: A. Peak to peak
Up: 8.2 Radicals containing r
Previous: 8.2.10 Rb(I) complex with
Contents
8.2.11 Nitrobenzene anion radical [a031]
Now the unpaired electron is dislocated preferably in the nucleus of nitrogen
and in positions ortho, meta and para of the aromatic ring.
We have, therefore, three triplets and a doublet, i.e. four different splittings
observed in the experimental spectrum.
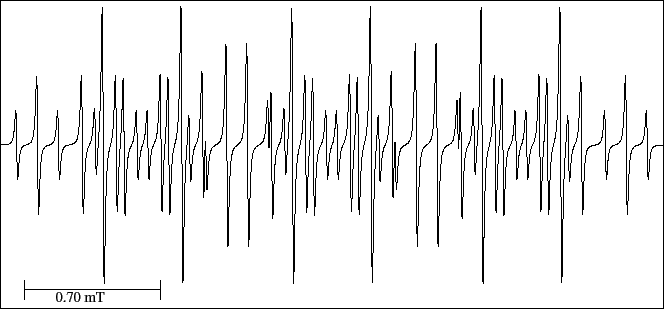
The spectrum (Fig. 39) has 50 lines while its maximum number is 54;
4 lines (two in each side of its centre) overlap in position with the splitting of two different triplets.
Observe the six lines of equal height situated symmetrically and if we joint them by couples
they form three doublets (6-13, 22-29 and 33-40);
the centres of the three doublets form a triplet that gives us the greatest hyperfine splitting.
[I] Measure the splittings. Proceed as in the example [a030] (subsection 8.2.9),
that is, reduce the range of the spectrum (between -1.7 and 0.0 mT).
Figure 39:
EPR spectrum of the nitrobenzene anion radical.
 |
[Exercise]
- Print
the following
- Measure the heights of the lines 1, 2, 6, 17 and 18 and normalize them.
Verify that they follow the relative theoretical intensities: 1:2:4:3:3.
- Print the simulated spectrum with the tree of splittings.
- Write down the lines that form the three doublets.
- Write down the lines of the six triplets that arise from the splittings of the three doublets
- Indicate the 4 lines that arise from of these triplets that do not follow the relation 1:2:1.
- Write in the form of results the different canonical structures of the anion radical and order
them according to its stability.
- Number the radical and assign the hyperfine splittings considering the previous section.
- Fill the Table Tab-a031.
Appendix A. The peak to peak linewidth (DHpp)




Next: A. Peak to peak
Up: 8.2 Radicals containing r
Previous: 8.2.10 Rb(I) complex with
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada