Next: 8.2.10 Rb(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.8 Rb(I) complex with
Contents
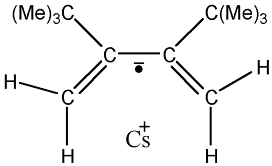
8.2.9 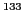 Cs(I) complex with 2,3-di-tert-butyl 1,3-diene anion radical [a030]
Cs(I) complex with 2,3-di-tert-butyl 1,3-diene anion radical [a030]
This ionic-pair formed by an anion radical associated with the cation 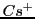 presents 72 experimental
lines (Fig. 36).
The caesium with atomic mass number 133 has a natural abundance of the 100% and a value of I = 7/2;
therefore, its coupling with the unpaired electron would give eight lines, octet
presents 72 experimental
lines (Fig. 36).
The caesium with atomic mass number 133 has a natural abundance of the 100% and a value of I = 7/2;
therefore, its coupling with the unpaired electron would give eight lines, octet
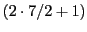 ,
of equal height (intensity).
In the experimental spectrum the following points are observed:
,
of equal height (intensity).
In the experimental spectrum the following points are observed:
- a)
- The lines 1-2-4 form a triplet with intensities 1:2:1.
- b)
- The lines 2-5-8 form another triplet, with intensity double of that of the previous one.
- c)
- Eight lines of equal height and constant separation, 5-14-23-32-41-50-59-68.
These experimental facts suggest that:
1) we have two splitting values very close that correspond to two groups with two hydrogen atoms,
positions 1,4-exo and 1,4-endo of the butadieno, respectively;
2) the eight lines of the spectrum are due to the Cs, and this splitting is much higher
than that of the triplets;
3) The hyperfine splitting owed to the 18 equivalent H of Terc-butyl is not detected.
[I] To measure the hyperfine splittings specially those of the triplets
change the range of the experimental spectrum (from -10.20 to 0 mT).
Compare the simulated with the experimental spectrum and refine the parameters until they overlap correctly.
Figure 36:
EPR spectrum of the 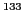 Cs(I) complex with 2,3-di-tert-butyl 1,3-diene anion radical.
Cs(I) complex with 2,3-di-tert-butyl 1,3-diene anion radical.
 |




Next: 8.2.10 Rb(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.8 Rb(I) complex with
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada