Next: 8.2.9 Cs(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.7 1,4-dihydro pyrazine cation
Contents
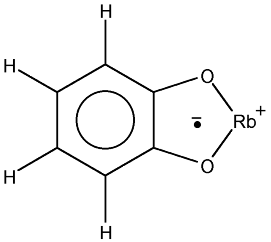
8.2.8 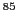 Rb(I) complex with 1,2-benzosemiquinone anion radical [a029]
Rb(I) complex with 1,2-benzosemiquinone anion radical [a029]
If we only consider the equivalent positions 1,4 and 5,6 in the aromatic ring,
the total number of lines would be 9.
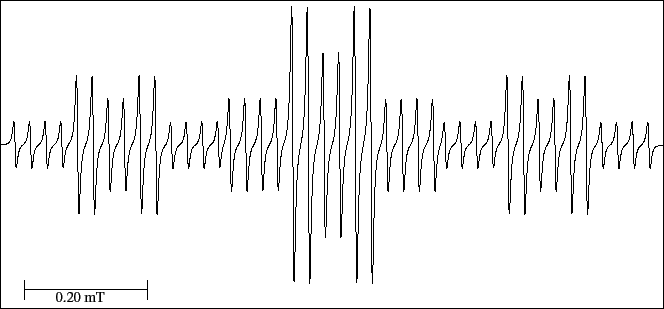
Nevertheless, the number of experimental lines for the spectrum of the Fig. 35 is 42.
This suggests that the nucleus of Rb with 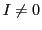 couples also with the unpaired electron.
The Rb has two isotopes whose atomic mass number numbers are 85 and 87 and with
spin quantum number of 5/2 and 3/2, respectively.
Its coupling with the electron would give
couples also with the unpaired electron.
The Rb has two isotopes whose atomic mass number numbers are 85 and 87 and with
spin quantum number of 5/2 and 3/2, respectively.
Its coupling with the electron would give 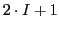 lines of equal intensity.
The result would be a sextet (1:1:1:1:1:1) or a quartet (1:1:1:1)for
lines of equal intensity.
The result would be a sextet (1:1:1:1:1:1) or a quartet (1:1:1:1)for 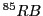 and
and 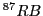 , respectively.
Applying the Eq. (9) we obtain a maximum number of lines for this anion radical
of 54 or 36 for each isotope.
In the spectrum, we see that the four first lines are equally spaced and they are of the same height.
The fact that the total number of lines is higher than 36 indicates that
the isotope that interacts is that of I=5/2.
The smallest splitting corresponds to the
, respectively.
Applying the Eq. (9) we obtain a maximum number of lines for this anion radical
of 54 or 36 for each isotope.
In the spectrum, we see that the four first lines are equally spaced and they are of the same height.
The fact that the total number of lines is higher than 36 indicates that
the isotope that interacts is that of I=5/2.
The smallest splitting corresponds to the 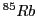 and
the successive splitting would be a triplet of triplets of sextets.
Consequently, we have 54/6 = 9 sextets whose distance between the 1
and
the successive splitting would be a triplet of triplets of sextets.
Consequently, we have 54/6 = 9 sextets whose distance between the 1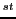 and the 2
and the 2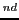 line would be the
splitting of the
line would be the
splitting of the 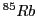 .
The experimental spectrum has a number of lines (42) smaller than that predicted (54),
because there are six lines situated symmetrically to each side of the centre of the
spectrum that add their intensities (overlap) with the lines of other sextet.
Detect in the experimental spectrum these 12 lines, measure (with the mouse)
their height and taking as reference the first one calculates the relation
of heights and compare with their theoretical relative intensities.
.
The experimental spectrum has a number of lines (42) smaller than that predicted (54),
because there are six lines situated symmetrically to each side of the centre of the
spectrum that add their intensities (overlap) with the lines of other sextet.
Detect in the experimental spectrum these 12 lines, measure (with the mouse)
their height and taking as reference the first one calculates the relation
of heights and compare with their theoretical relative intensities.
Figure 35:
EPR spectrum of the 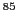 Rb(I) complex with 1,2-benzosemiquinone anion radical.
Rb(I) complex with 1,2-benzosemiquinone anion radical.
 |
[Exercise]
- Measure with the mouse the heights of the lines of the spectrum that overlap.
- Print and fill
the following form:
- Reconstruct the spectrum by successive splittings in the graph paper.
- Mark with an arrow the lines of the spectrum that overlap with those that coming forma other sextet.
- Deduce the relative theoretical intensities (takes as reference the line 1).
- Compare the values obtained with the normalized experimental heights.
- Do the transformation from cm to mT and find out the hyperfine splittings.




Next: 8.2.9 Cs(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.7 1,4-dihydro pyrazine cation
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada