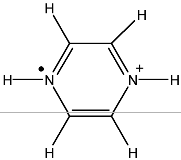
Next: 8.2.8 Rb(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.6 Mono-deuterium diethyl amine
Contents
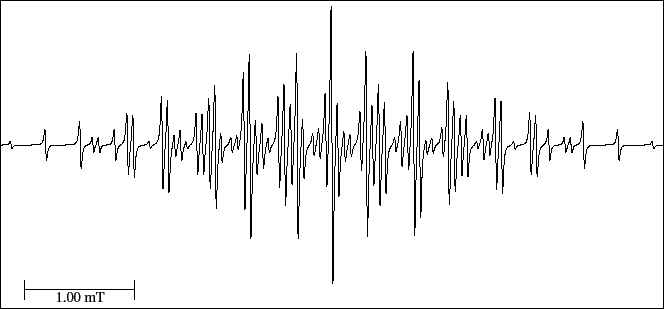
Figure 34:
EPR spectrum of the 1,4-dihydro pyrazine cation radical.
 |
[Exercise]
- Using the symmetry of the radical, deduce the number of equivalent groups.
- Counts the number of experimental lines; calculate the number of theoretical lines (Eq. (9));
write down both values and observe the difference.
- Measure (with the mouse) the heights of the three first lines of the spectrum.
From these heights, deduce if the smaller splitting comres from the two atoms of N or from the four equivalent H
of the ring, since both would give a quintet but with different relation of intensities.
- Measure the distance between lines 2 and 3.
This distance is the same than that between the lines 1 and 2.
- Seek the lines that form the first quintet and from the line 4, with the distance 1-2
find the 2
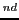 quintet.
Write down the lines that form the first and the second quintet of the spectrum.
quintet.
Write down the lines that form the first and the second quintet of the spectrum.
- The distance among the central lines of the previous quintets (lines 3 and 10)
is the second hyperfine splitting (it can also be measured among the lines 1-4).
- With the distance among the lines 3-10, find the following multiplet (triplet or quintet?);
Write down the lines of that multiplet.
- Measure the length of the experimental spectrum and apply the Eq. (8)
to calculate the third hyperfine splitting.
- From the centre of the spectrum (line 35), write down the lines generated
when the third splitting is applied.
- Measure the heights of all the lines to determine the order of the hyperfine splitting.
- In the results paper printed with the simulator, number the nuclei of the radical
and assign the hyperfine splittings to each nucleus showing the positions that are equivalent.
- Print and fill
the following
for the radical interpretation.
[I] In this spectrum the value given to DHpp is very important, since the two largest constants
are very close.
If we do not reduce the DHpp, the spectrum can be correctly interpreted but there will be less
lines than in the experimental one.




Next: 8.2.8 Rb(I) complex with
Up: 8.2 Radicals containing r
Previous: 8.2.6 Mono-deuterium diethyl amine
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada