



Next: 8.2.4 Bis (tri-fluoro methyl)
Up: 8.2 Radicals containing r
Previous: 8.2.2 Di-nitro methane anion
Contents
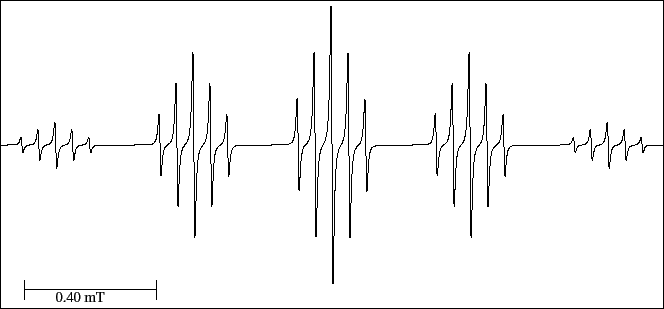
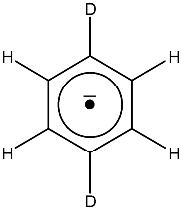
The spectrum of Fig. 30 presents a quintet of quintets originated by the coupling of the
unpaired electron with the two equivalent atoms of deuterium and with the four equivalent atoms of hydrogen.
We have said (see Note in subsection 8.2.1) that within the same chemical environment,
the hyperfine splitting of a proton is 6.5 times larger than that of deuterium, therefore,
it is foreseeable that in this spectrum, the larger splitting corresponds to the four protons and
the smaller one to the two deuterium atoms.
Nevertheless, in this case, the measure of the heights of the lines of each quintet in the
experimental spectrum allows to assign the splittings.
Figure 30:
EPR spectrum of the 1,4-di-deuterio benzene anion radical.
 |
[Exercise]
Measure the heights of the lines of the 2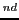 quintet and fill out the corresponding rows of the
Intensities Table 2,
which you already printed in the section 8.1.5.
Observe the difference in the intensities of the signals.
Do not forget to attach this Table in the laboratory notebook.
quintet and fill out the corresponding rows of the
Intensities Table 2,
which you already printed in the section 8.1.5.
Observe the difference in the intensities of the signals.
Do not forget to attach this Table in the laboratory notebook.




Next: 8.2.4 Bis (tri-fluoro methyl)
Up: 8.2 Radicals containing r
Previous: 8.2.2 Di-nitro methane anion
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada

quintet and fill out the corresponding rows of the Intensities Table 2, which you already printed in the section 8.1.5. Observe the difference in the intensities of the signals. Do not forget to attach this Table in the laboratory notebook.