Next: 8.2.5 Diethyl amine cation
Up: 8.2 Radicals containing r
Previous: 8.2.3 1,4-di-deuterio benzene anion
Contents
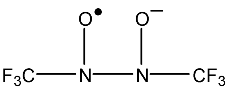
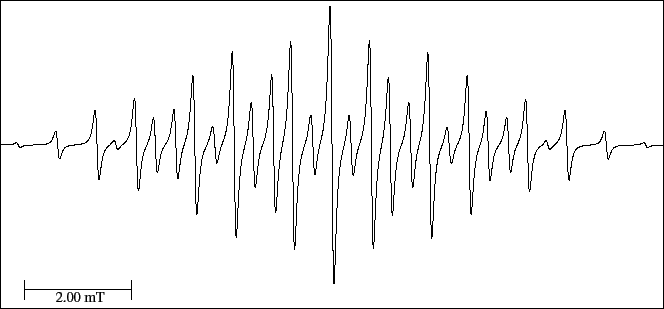
For this radical a maximum of 35 lines is obtained (Eq. (9)), nevertheless,
the experimental lines (Fig. 31) are 29.
There are three lines to each side of the central line that coincide with the splitting of two multiplets.
We have two possibilities: 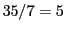 septets or
septets or 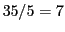 quintets, depending on that
quintets, depending on that 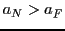 or
or 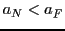 .
Measure the two splittings in the experimental spectrum,
simulate the two possibilities and note down the differences.
Observe the reconstruction by successive splittings of the simulated spectrum and detect which six lines
of the experimental spectrum add its intensities (overlap).
.
Measure the two splittings in the experimental spectrum,
simulate the two possibilities and note down the differences.
Observe the reconstruction by successive splittings of the simulated spectrum and detect which six lines
of the experimental spectrum add its intensities (overlap).
Figure 31:
EPR spectrum of the Bis (tri-fluoro methyl) semidiazoxide anion radical.
 |
[Exercise]
- Print
the following
- Carry out two simulations: the first one with
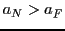 (simulation 1)
and the second with
(simulation 1)
and the second with 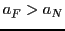 (Simulation 2).
(Simulation 2).
- Print both simulations with the tree of successive splittings.
- Measure the length and the number of lines of the two simulated spectra and write down these values in the Table-a025.
- Assign a number to each line of the spectrum simulated-1 and mark on it the central lines of the five septets
that give place to the quintet originated by the coupling with the nitrogen.
Measure and write the Table the heights of these five lines.
- Assign a number to each line of the spectrum simulated-2 and mark on it the central lines of the seven quintets
that will give a septet originated by the coupling with the fluorine.
Measure and write in the Table the heights of these seven lines.
- Complete the Table and say which of the two simulations is more close to the experimental spectrum.
- Enclosed the result in the laboratory notebook.




Next: 8.2.5 Diethyl amine cation
Up: 8.2 Radicals containing r
Previous: 8.2.3 1,4-di-deuterio benzene anion
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada