



Next: 8.2.6 Mono-deuterium diethyl amine
Up: 8.2 Radicals containing r
Previous: 8.2.4 Bis (tri-fluoro methyl)
Contents
8.2.5 Diethyl amine cation radical [a026]
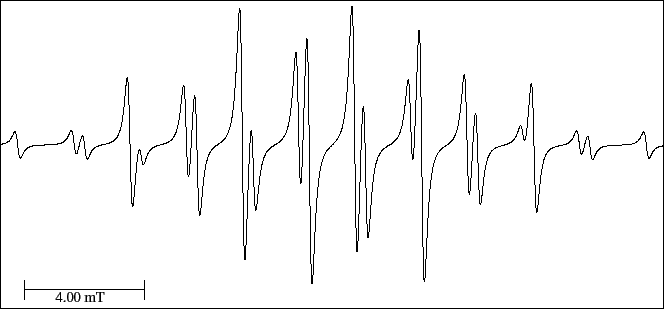
According to the molecular structure, the maximum number of lines would be 210 while in
the experimental spectrum (Fig. 32) there are only 22.
This can be explained because the splitting for the hydrogen of the methyl groups is very
small and is not observed in the spectrum.
Therefore, we have only three hyperfine splittings.
Find out the three splittings applying the basic interpretation rules (section 7).
Figure 32:
EPR spectrum of the diethyl amine cation radical.
 |
[Exercise]
- Carry out two simulations: the first one with
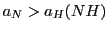 (Simulation 1) and the second
with
(Simulation 1) and the second
with 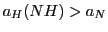 (Simulation 2).
(Simulation 2).
- Print the correct simulation with the spectra without overlap and including the tree of successive splittings.
- Show on the tree of successive splittings the relative theoretical intensities of each multiplet.
The interpretation of the radical [a024] (section 8.2.1 and Fig. 27) can help you with this problem.
- Mark with an arrow the lines of the spectrum that add their intensities and
indicate their relative theoretical intensity.
[Exercise]
- Knowing that
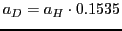 , do the simulation of the spectrum that
would be obtained for the molecule
, do the simulation of the spectrum that
would be obtained for the molecule
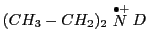 (Simulation-D).
(Simulation-D).
- Print the final simulated spectrum.
- Fill out the




Next: 8.2.6 Mono-deuterium diethyl amine
Up: 8.2 Radicals containing r
Previous: 8.2.4 Bis (tri-fluoro methyl)
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada
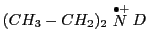 (Simulation-D).
(Simulation-D).