The spectrum of Fig. 4 corresponds to the
![]() radical.
The multiplicity is due to the two equivalent methylic protons that give a triplet (see Table 2).
The proton of the hydroxyl group exchanges rapidly with the solvent (
radical.
The multiplicity is due to the two equivalent methylic protons that give a triplet (see Table 2).
The proton of the hydroxyl group exchanges rapidly with the solvent (![]() , pH = 1)
and, therefore, its hyperfine splitting cannot be detected
(we can consider that the hyperfine splitting is null).
, pH = 1)
and, therefore, its hyperfine splitting cannot be detected
(we can consider that the hyperfine splitting is null).
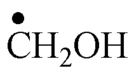
The intensity (height) of the signals or peaks can be measured easily in the simulator by clicking with the mouse on the button and on the top of the peak. The intensity will be shown in ''Dist-Y'' in units of pixels. If we measure the intensity of the peaks of a multiplet and divide for the intensity of the smaller one, we will get the normalised intensities (see Tables 2 y 3).
[Exercise] Print out the following Table
Measure the intensities in the simulator and fill the rows corresponding to the spectrum [a001]. Further on you will measure the intensities of the spectra [a003], [a007], [a008], [a009] and [a010]. Observe the difference in the intensities of the signals with the same multiplicity. Do not forget to attach this Table in the laboratory notebook.