Next: 8.1.11 Benzyl neutral radical
Up: 8.1 Radicals containing r
Previous: 8.1.9 Anthracene anion radical
Contents
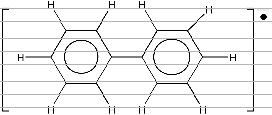
8.1.10 Biphenyl anion radical [a020]
In this molecule, the unpaired electron is dislocated in the ortho, meta and para positions of the ring giving
three groups of equivalent hydrogen.
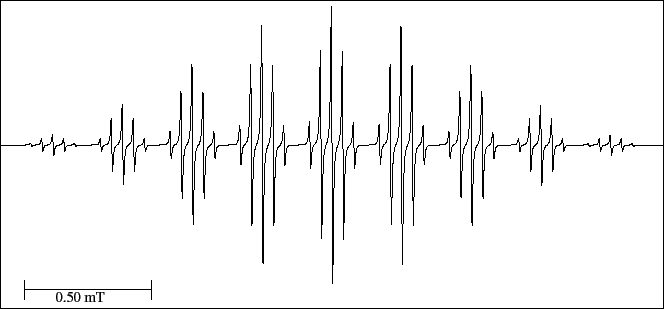
Nine quintets are observed in the spectrum (Fig. 25) which originate
45 experimental lines, a number smaller than the predicted one,
 lines.
This is because the value of the triplet (positions 4,4') hyperfine splitting is double
of that of the quintet (positions 2,2',6,6').
If the situation were the reverse, the spectrum will have 11 quintets and therefore 55 experimental lines.
lines.
This is because the value of the triplet (positions 4,4') hyperfine splitting is double
of that of the quintet (positions 2,2',6,6').
If the situation were the reverse, the spectrum will have 11 quintets and therefore 55 experimental lines.
Figure 25:
EPR spectrum of the biphenyl anion radical.
 |
[Exercise]
Keeping in mind the previous observations:
- Load the EPR simulator.
- Verify the horizontal scale with the mouse and measure the three hyperfine splittings.
- Measure the heights (with the mouse) of the 9 central lines of each quintet and
calculate the relation of heights taking as reference the first one.
Compare with the theoretical intensities 1:4:8:12:14:12:8:4:1.
- Use, as additional data, the total length of the experimental spectrum
and the value of DHpp (measured using a smaller range) to overlap
correctly both spectra.
- Print out the results.
[Exercise]
- Fill out again the table of splittings on the simulator but changing the order of the two larger constants,
that is,
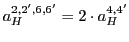 .
Simulate the new spectrum (simulation 2).
.
Simulate the new spectrum (simulation 2).
- Measure the heights of the central lines of each quintet and the total length of the spectrum.
- Print out
the following:
- Complete the Table with the intensities measured for both spectra.
- Enclose the result in the laboratory notebook.




Next: 8.1.11 Benzyl neutral radical
Up: 8.1 Radicals containing r
Previous: 8.1.9 Anthracene anion radical
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada